Water performs many vital functions, but not all of its properties provide benefits. Naturally hard water contains dissolved calcium and other minerals which help to build and maintain healthy bodies, but their effect on water systems can be very damaging.
 When dissolved minerals, such as calcium bicarbonate, revert to their solid carbonate state, limescale is formed in water systems and this impedes heat transfer, narrows pipes and blocks sprays. Not only does this dramatically reduce system efficiency but it provides a breeding ground for bacteria, such as Legionella.
When dissolved minerals, such as calcium bicarbonate, revert to their solid carbonate state, limescale is formed in water systems and this impedes heat transfer, narrows pipes and blocks sprays. Not only does this dramatically reduce system efficiency but it provides a breeding ground for bacteria, such as Legionella.
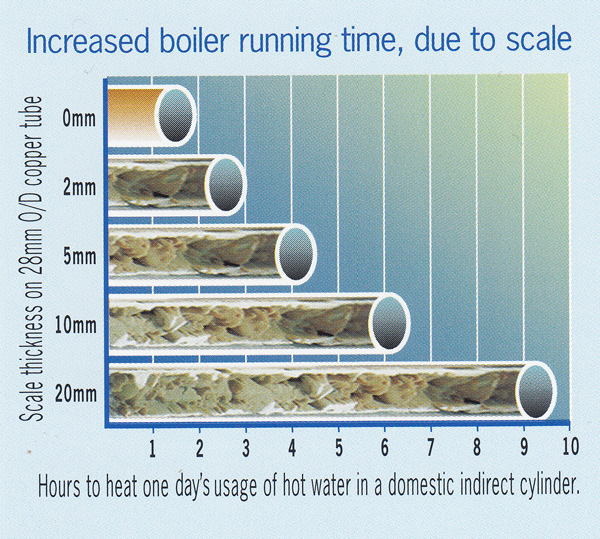
Research has shown that just 3 mm of limescale can reduce energy efficiency by a staggering 21%, and, in a moderately hard water area this can form on heat exchangers in just six months! £Billions are wasted each year in increased energy costs, lost production and early renewal of damaged equipment and appliances.
With a clean heat exchanger, a typical domestic hot water calorifier takes 1½ hours to heat a family’s water. With just 2 mm of scale the boiler has to run for another hour to heat the water, wasting a significant amount of fuel. (Source: University of Portsmouth)